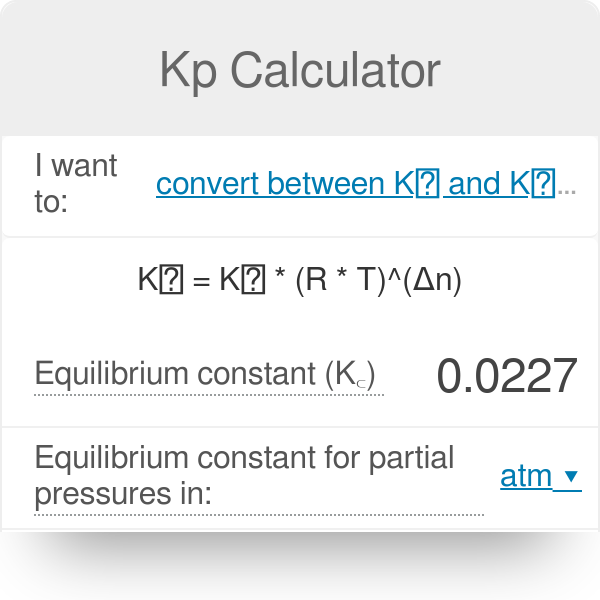
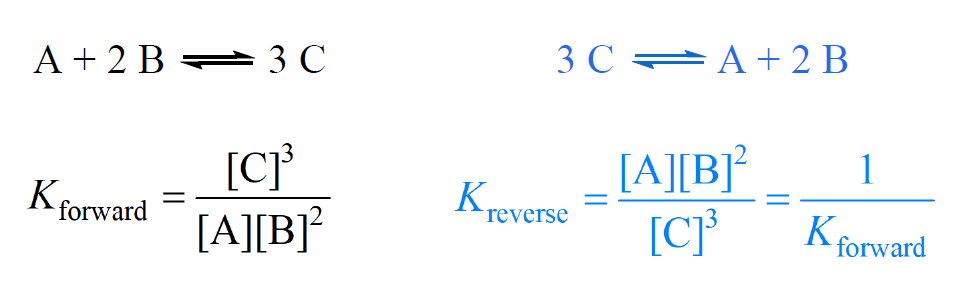
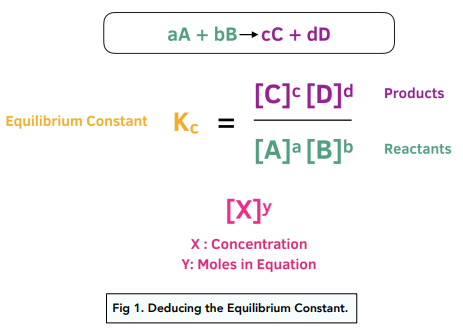
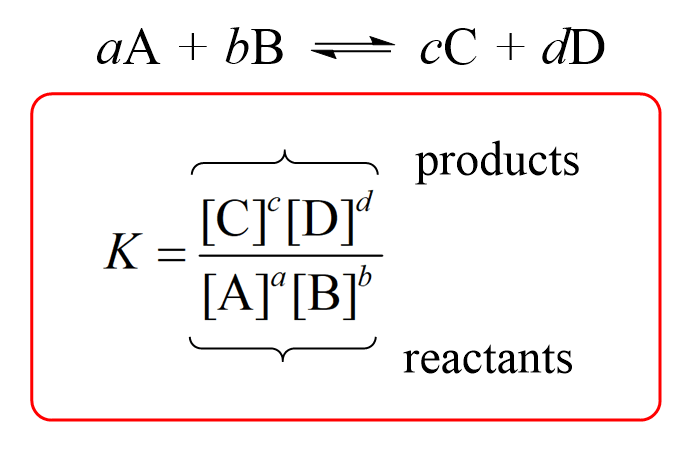
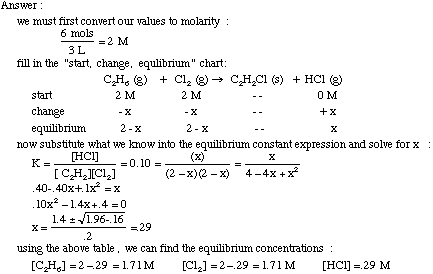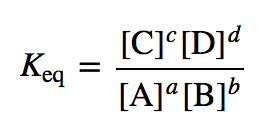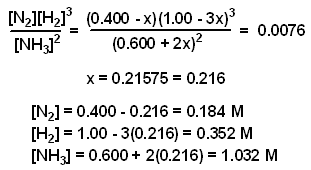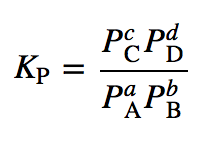Calculate the equilibrium constant for the reaction at 25∘ C. Fe + CuSO 4⇌ FeSO 4+ CuGiven EO Pi z0=0.44 V ; EO PLu0=0.337 VA. 10+26.33B. 10–20.69C. 10+20.69D. 10–26.33

Easy tricks to calculate equilibrium constant based problems/Chemical eq... | Simple tricks, Equilibrium, Trick

Calculating equilibrium constants from equilibrium concentrations or partial pressures (worked examples) (video) | Khan Academy

SOLVED: Calculate the equilibrium constant for the following reaction at 25°C: Zn2+(aq) + 2Ag(s) ⇌ Zn(s) + 2Ag+(aq) Round your answer to one significant figure. Enter your answer in scientific notation. Note,

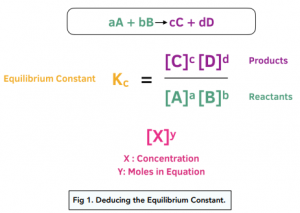
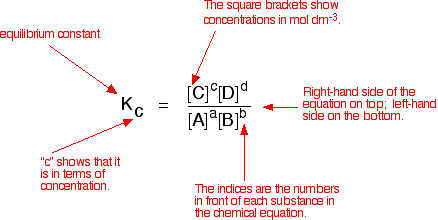
Learn how to calculate an equilibrium constant Kc. | Chemistry lessons, Teaching chemistry, Chemistry education
Calculate the equilibrium constant for the redox reaction at 25°C. Sr(s) + Mg^(2+) → Sr^(2+)(aq) + Mg(s), - Sarthaks eConnect | Largest Online Education Community
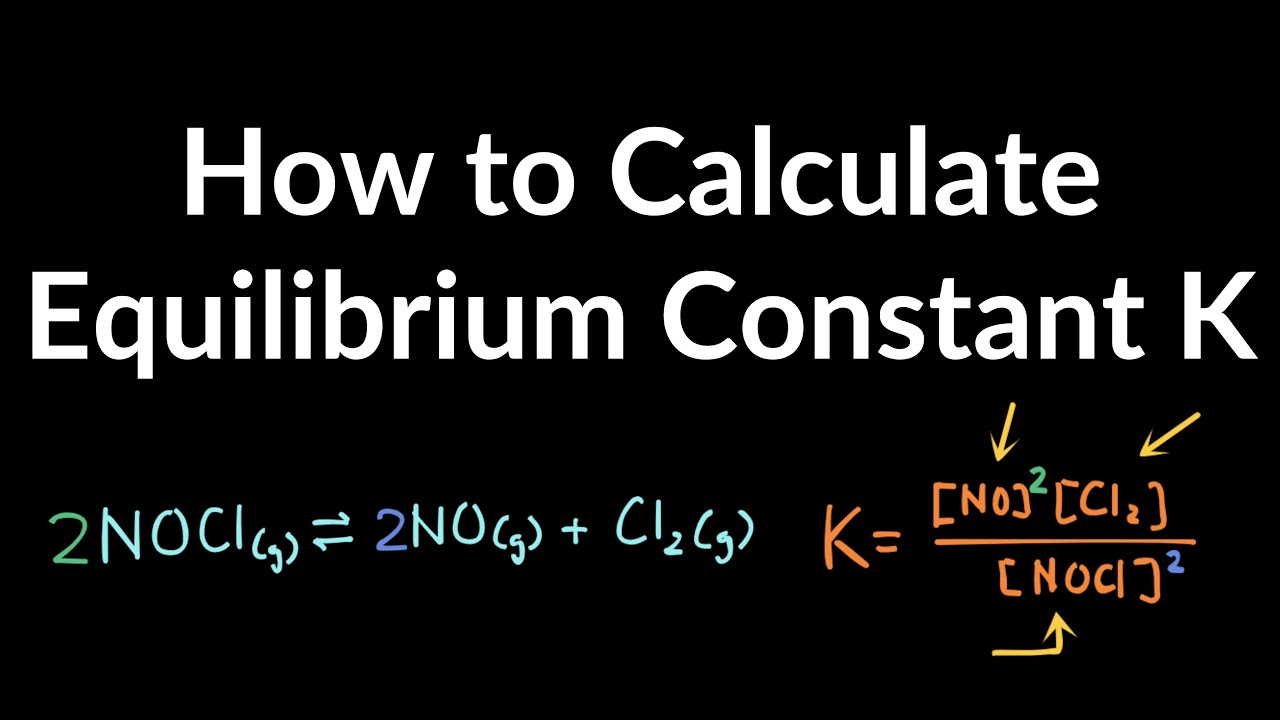
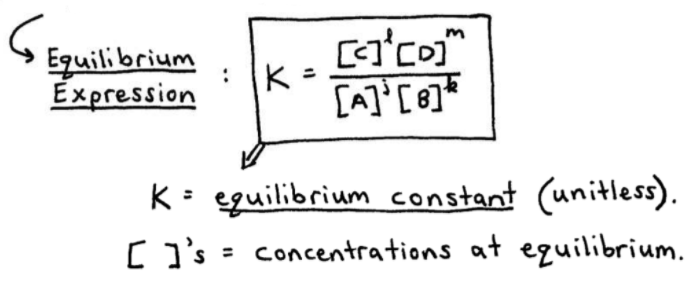
How to Calculate Equilibrium Constant K Value Practice Problems & Exampled Explained Step by Step - YouTube